Why Do Alkali Metals and Halogens React
Halogens are so reactive because they have 7 valence electrons and are very close to having a complete shell of 8 electrons. The Halogens - Reactions with Metals.

Chemical Reaction Images Chemical Reactions Chemistry Lessons Science Chemistry Law Of Multiple Proportions
Why does Group 7 get less reactive.

. Reactivity decreases down the group. The noble gases have filled valence shells as they occur in nature. 2K H 2 SO 4 K 2 SO 4 H 2.
Exposing an alkali metal to a halogen will cause an extremely exothermic reaction that results in an ionic salt. Because they have only one valence electron group 1 elements are highly reactive. Group 1 metals - see the reaction with alkali metals.
In this way why is Group One called alkali metals. It is this electrostatic attraction that forms ionic bonds in metal halides and other ionic compounds. The halogens are reactive non-metals.
The group 1 of the periodic table contain six elements namely LithiumLi SodiumNaPotassiumKRubidiumRbCesiumCs and FranciumFr. Why do alkali metals and halogens react so strongly with other elements. The alkali metals are more reactive than alkaline earth metals as alkali metals have just one valence electron and this makes atoms more reactive.
Almost every salt of an alkali metal is highly soluble in water. Therefore they easily lose this electron to. Why do halogens react with metals.
This is due in part to their larger atomic radii and low ionization energies. Why do ions with a 1 charge react in a 1 1 ratio with halogens. The halogens all have the general electron configuration ns 2 np 5 giving them seven valence electrons.
They tend to donate their electrons in reactions and often have an oxidation state of 1. What state of matter is sodium. Start studying Halogens and Alkali Metals GCSE.
A full octet of. How do the Halogens React with Metals. Reactions with oxygen The alkali metals forms ionic solids where the alkali metal has an oxidation number of 1.
Do halogens react with alkali metals. The reaction is less than the other alkali metals with water oxygen and halogens and the reaction is more with nitrogen carbon and hydrogen. The alkali metals are soft reactive metals.
Alkali metals have a very low ionization energy which allows them to easily lose an electron but halogens have a very high electronegativity which allows them to easily absorb an electron. The Group 7 elements are known as the halogens. Why is hydrogen in a group of reactive metals.
Alkali metals and halogens do not occur in free state in nature because they are very reactive. The term halogen means salt-former because these elements will readily react with alkali metal and alkaline earth metals to form halide salts. The alkali metals and their compounds are found in abundance in both nature and everyday life.
Halogens react with alkali metals to form salts. The halogens will react with. Why are halogens so reactive.
Hydrogen and alkali metals reaction. Almost every salt of an alkali metal is highly soluble in water. When the alkali metals react with the different halogens Group 7 of the periodic table the group of compounds formed are known as the alkali metals halides.
2Na H 2 2NaH. 2Na 2HCl 2NaCl H 2. How does alkali metals react with halogens.
Learn vocabulary terms and more with flashcards games and other study tools. They react vigorously with water and become more reactive as you go down the group. Halogens want to lose a valence electron.
They form conducting solutions proving their ionic nature. Alkali metals react vigorously with halogens. They are reactive non-metals and are always found in compounds with other elements.
Transition metals - for example. The alkali metals halogens and noble gases are three important groups in the periodic table. The electron structure of halogens means that they react vigorously with group 1 alkali metals.
Strong bases capable of neutralizing acids when. Halogens are very reactive because they have seven valence electrons and need one more to have eight valence electrons an octet. Alkali metals are known for being some of the most reactive metals.
This is because alkali metals have 1 electron in. These metals are characterized as being extremely soft and silvery in color. Due to the fact that there are a.
The positive alkali metal ions and the negative halide ions are strongly attracted to each other. These metals are called alkali metals because they form alkalies ie. Group 1 of the periodic table includes hydrogen and the alkali metals.
It resembles alkali metals in some of its properties but it also resembles halogens in its properties. Alkali metals all have a single valence electron and halogens have a single missing one so they would combine in a 11 ratio. The combining ratio for alkali metals and halogens is 11.
Group 2 metals - see the reaction with magnesium and calcium. What do sodium and chlorine make when combined. Is sodium reactive or.
Exposing an alkali metal to a halogen will cause an extremely exothermic reaction that results in an ionic salt. Alkali metals react with strong acids HCl HNO 3 H 2 SO 4 and emit hydrogen gas and produce relevant alkali metal salt. Chlorine bromine and iodine are all halogens.
They form conducting solutions proving their ionic nature. Helium has a duet of valence electrons and the rest of the noble gases have an octet. Acids and alkali metals reaction.
This happens because alkali metals have one electron in their outer shell that they want to give away and halogens have seven electrons in their outer shell and they want to gain one electron. Metal hydrides H- are given as products. The halogens will gain one electron to form an ionic bond when they react with metals.
They are unstable and react with other elements to form compounds in order to attain stability. Those ionic hydrides have H-ions. Ionic compounds are neutral so the total positive charge has to equal the total negative charge.
Why do alkali metals and halogens like to Chemical bond. The laws of electrons and atoms on a basic level are governed by thermodynamic stability this is not the only thing at play here but it is the most obvious and easiest to understand to suit our needs. What do alkali metals react vigorously with.

Element Infographics The Alkali Metals Poster By Compound Interest In 2022 Alkali Metal Element Chemistry Chemistry Lessons
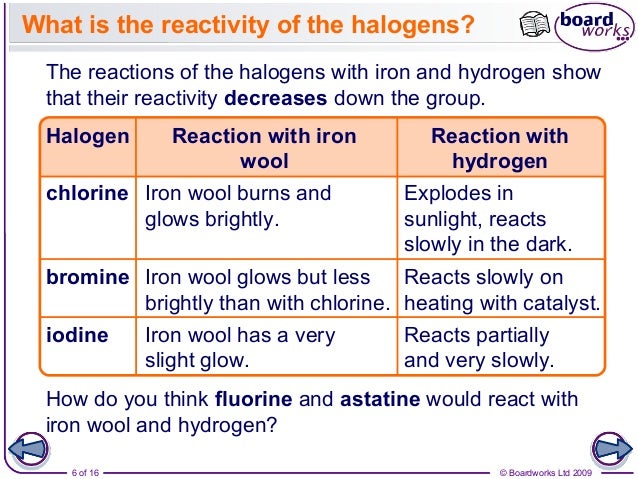
Comparing Halogen Reactivity Trends With Those Of The Alkali Metals Halogens Become Less Reactive Down The Chemistry Classroom High School Chemistry Chemistry

An Improved 4d Periodic Table Arranged Periodic Table Alkali Metal Carbon Nanotube

Periodic Table Alkali Metals Group 1a Alkaline Metals Group 2a Transition Metals Group B Metalloids 7 Purp Transition Metal Periodic Table Physical Chemistry

Halogen Easy Science Study Skills Halogen Periodic Table

Element Infographics The Alkali Metals Poster By Compound Interest Alkali Metal Chemistry Lessons Element Chemistry

Free Periodic Table Of Elements Color By Category Periodic Table Of The Elements Informational Texts Activities High School Science

Alkali Metals Properties Periodic Table Ios App Chemistry Periodic Table App

Sodium And Halogens Explosive Reactions Chlorine Bromine Iodine Iodine Chlorine Chemistry

Image Result For Periodic Table With Alkali Metals Alkaline Earth Metals Halogens And Noble Gases Cetvel Kimya Egitim Felsefesi

Active Metals The Alkali Metals And Alkaline Earth Metals Most Easily Oxidized So React Most Readil Chemistry Education Homeschool Science Transition Element

Alkaline And Alkaline Earth Metals Alkaline Earth Metals Alkaline Chemistry Education

This Periodic Table Shows How Much We Interact With Each Element Chemistry Help Teaching Chemistry Chemistry Classroom

Bbc Gcse Bitesize Groups Gcse Chemistry Periodic Table Chemistry

Internet Database Of Periodic Tables Chemogenesis Internet Database Periodic Table Teaching Chemistry

Ks4 Chemistry Halogens Additional Science Chemistry Physical And Chemical Properties

Collective Names Of Like Elements Chemicalelements Periodictable Halogens Pnictogens Chalcogens Alkali Chemistry Education Chemistry Notes Physics Notes

Comparing Halogen Reactivity Trends With Those Of The Alkali Metals Halogens Become Less Reactive Down The Chemistry Classroom High School Chemistry Chemistry

Comments
Post a Comment